|
||||
| The Angio Institute | Research & Education | Brain Health Program | AngioNews/Media | Joining Hands |
When considering the purchase of any health or wellness device, today’s health professional and consumer are far more demanding of deeper answers about innovations. With high speed access to web research are at our fingertips, cutting through the jungle of marketing ads and product hype (to access proven science and technical data) is part of our info culture for the ‘smarter tech shopper’. Thanks to web research, we can make far better choices thanks to user reviews, company background checks, case studies or testimonials. HEALTH TECH REPORTER, an allied partner of the AngioFoundation presents a clinical perspective on health technologies from seasoned and unbiased testers and performance examiners to share their reviews for your added insight.
SCIENTIFIC REPORTING SUPPORTS Especially in the case of medical devices and health products, public interest for true quality and performance calls for a more critical eye, one that is not influenced by conventional sales and marketing methods. The value of a good review from AN OUTSIDE, INDEPENDENT TESTER (led by an uncompromised team) can offer a more confident understanding and technical credence about the product in question. It is the process of this type of ‘test drive’ that can offer valuable insight from actual clinical professionals. MEDTECH REVIEWS* aims to bring this unquiue evidence and SCIENCE based observation about the effects of the selected health innovations under review.
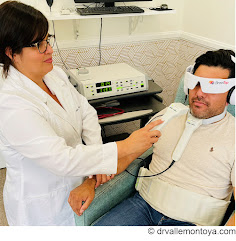
A MORE INVOLVED & UNBIASED TEST DRIVE EVIDENCE-BASED PERFORMANCE REPORTS •11/24 - Image Guided Approach to the Potency of PEMF on Prostate Hyperplasia (PDF report- official)
EFFICACY STUDIES & PUBLISHING
Our reporting is often designed to be small, private demo
reports and mini‐case studies offering ANECDOTAL
reports from professionals who provide both EDUCATION and AWARENESS about non‐invasive
technologies are the major objectives behind our reports. All our findings and reviews are
2) "OFF‐LABEL USE": Certain devices are accepted in our reports under the sole discretion of a certified diagnostic clinician for exploratory research if the concept of the technology protocol (not necessarily the specific model in hand) may show published potential response to manage or address other disorders that may have not yet earned FDA clearance or approval. Exploratory reviewing is completely academic in its nature, seeking to duplicate these experimental un‐cleared applications for any evidence of the pre‐published statements in the name of science.
3) OTHER EVALUATORS: Aside from Dr. Bard’s imaging, other volunteer
users of the product may be involved in the tech review experience, where 4) PUBLISHING FORMATS & DISTRIBUTION: The MedTech
review team shall submit their unbiased report, based solely on
their evaluation of the performance or effect(s) of the device in
review. These reviews can be in the form of a written review to
be published in our MedTech Newsletter for public access. This
newsletter can and will be shared throughout the networks of
our developers, including IPHA (Integrative Pain Healers
Alliance) and our list of health related organizations for re‐
posting/sharing. Also, our publishing team may produce ONE
VIDEO (usually 2‐3 minutes in length) of this review to be Feature Articles
5) TECH EVALUATION PROCESS: For any review to carry the
necessary validity, our professionals undergo specific protocols that conform to our reporting standards. We hand select
a team of health professionals who hold specific certifications that pertain to the device under review. For example, 6) ADVANCED REPORTING: As an educational function, the reporting initiative of MedTech Reviews* is not limited to the direct product testing of non‐invasive or portable devices. Our publishers are also committed to searching for the latest innovations and sharing direct insight on technologies that may be too large (or too involved) to test drive. This level of reporting is for major medical innovations or upgrades in health facilities. They are recognized as ground‐breaking technologies that shape the future of their designated functions or protocols. They are often hospital grade and oversized units or systems where our publishing team covers private interviews with their science officers & engineers.
|
|||||||||||||
 |
2023 Research: Exploratory Use of Extracellular Vesicles for CHRONIC Disorders |
||||||||||||
"SEEING IS BELIEVING": Advantages of Imaging in Research Studies
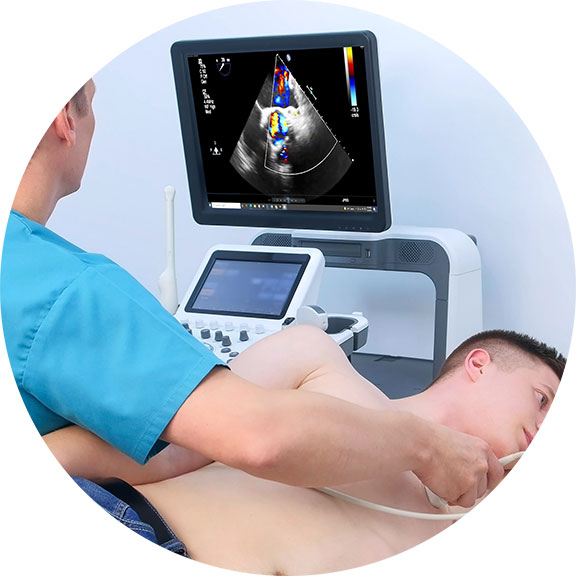
TRACKING THERAPEUTIC EFFECTS THROUGH BLOOD FLOW REACTIONS Throughout his career, Dr. Bard has employed this imaging strategy to detect, track and confirm the body's reaction to a variety of therapeutic interventions. He has conducted medical center based double-blinded, corporate sponsored and private studies reviewing the effects of injectable therapies (PRP, Stem Cell therapies, etc) as well as non-invasive therapeutic interventions in studies of neuro-stimulation, electrostimulation and electromagnetic field treatments. His approach involves the comparative study of measurable scanning data or quantitative ultrasound (QUS) which aims at recording interactions between the behavior and activity of biological tissue microstructure and ultrasound waves [5][6]. From a time-based comparative study of the treated area (before and after studies), Dr. Bard applies the use of blood flow detection technology or hemodynamic data gathering protocols, document specific objective and quantifiable biological responses to therapeutic treatments. Ref: |
|||||||||||||
"Before & After" Studies The most sensible and logical way to identify the results of any treatment is by tracking the body's response to it. Controlled testing must show the patient's condition PRE and POST effects, where true data-finding is collecting the necessary EVIDENCE of its claims. The investigator can pull a significant amount of data from this form of oberservational testing and recording: including stage-by-stage bodily response to future projections of possible side effects. Recording of any and all psysiological response means the researchers are counting on the patient's body to tell us what it is undergoing during the testing phase. To prevent mis-reading and erroneous reports, trials tend to work with a large number of test patients (commonly 50-100) and may also employ redundancies like undergoing multiple testing protocols for a second or even third opinion. To capture the benefits of a BEFORE AND AFTER review, Imaging is often used as a standard screening solution for the response of most of the major organs. |
|||||||||||||
|
|||||||||||||
| DEDICATED MONITORING OF PRE AND POST-TREATMENT PROGRESS Within a closed testing program of 45-50 patients, a comparative review of pre and post treatment allows the developer a clear view of its efficacy and performance. Through the use of various imaging technologies and the experienced assessment strategies of our diagnosticians, we are able to provide the desired data which leads the devleoper to identify quality standards as they apply to the inevitable end user en masse. |
Educational PROGRAMS
The AngioFoundation has been recognized worldwide by official medical organizations and peer reviews for its work in FIVE MAIN focal topics and class divisions. We continually maintain and update research works in these categories to support the advancement of these disciplines as part of The AngioFoundation's commitment to the scientific community.
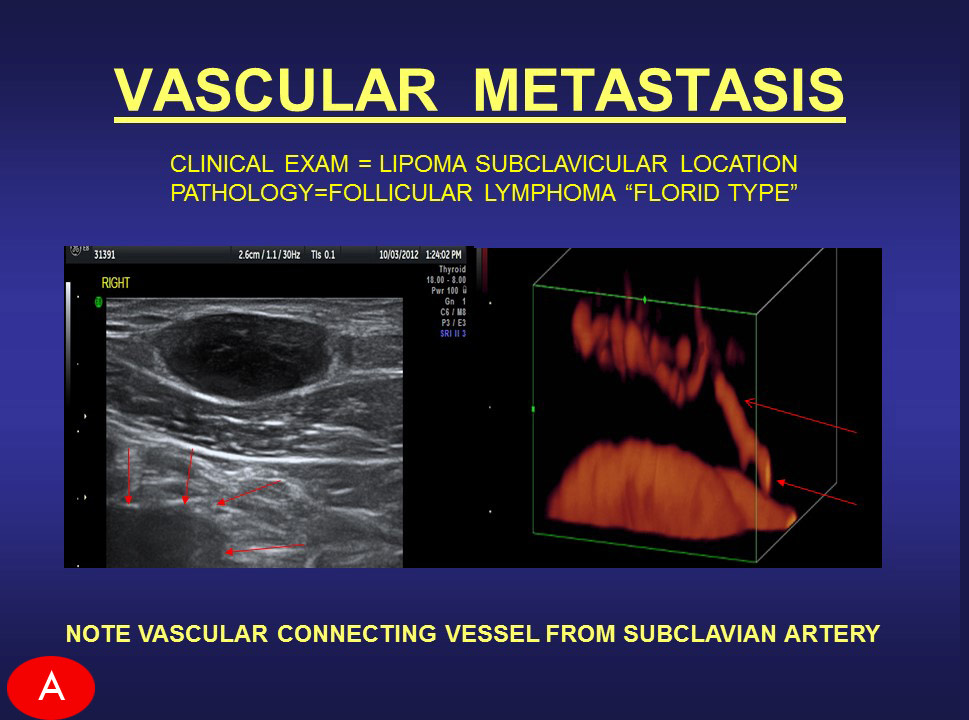
 Advanced use of Doppler Sonographic Imaging technology to identify malignant cancers and monitor their behavior through blood flow parameters- (Breast, Lung, Bladder, Prostate, Melanoma, etc) which correlates with comparative studies with other current technologies such as MRI, CT etc. Advanced use of Doppler Sonographic Imaging technology to identify malignant cancers and monitor their behavior through blood flow parameters- (Breast, Lung, Bladder, Prostate, Melanoma, etc) which correlates with comparative studies with other current technologies such as MRI, CT etc. |
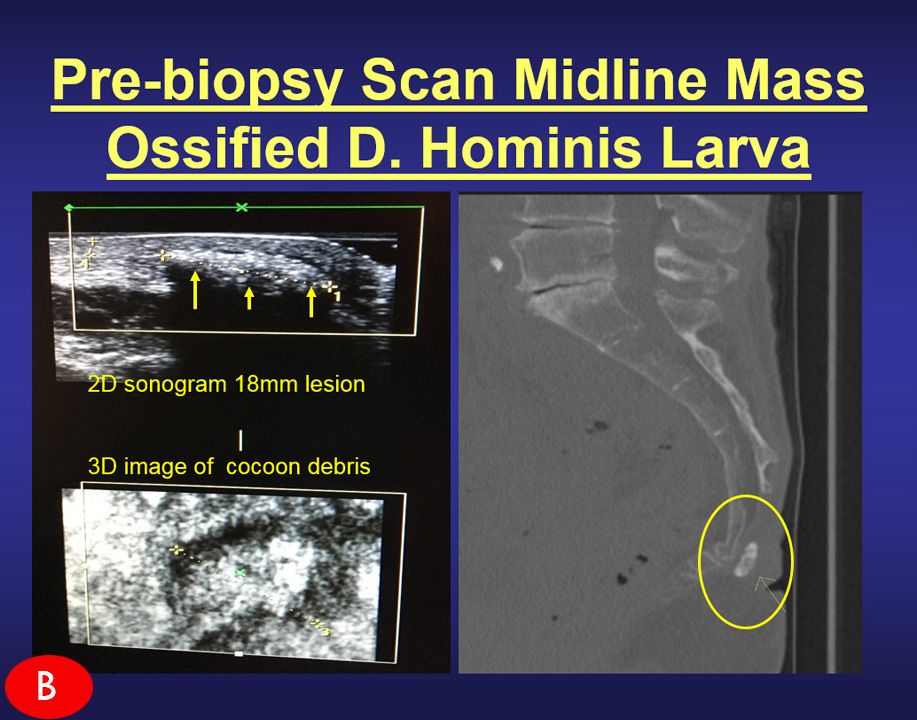
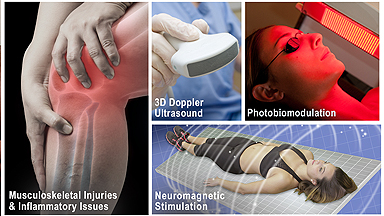
 Research / assessment of musculoskeletal disorders (arthritis, inflammation, trauma) and dermatological issues through the advanced use of 3D/4D ULTRASOUND innovations. Research / assessment of musculoskeletal disorders (arthritis, inflammation, trauma) and dermatological issues through the advanced use of 3D/4D ULTRASOUND innovations. |
 TECH REVIEWER: Beta-testing, industry-wide comparative feature review/evaluation program. Drafting of FDA application / compliance documentation of digital imaging technologies (models, brands and generations) including sub-dermal and musculoskeletal treatment devices TECH REVIEWER: Beta-testing, industry-wide comparative feature review/evaluation program. Drafting of FDA application / compliance documentation of digital imaging technologies (models, brands and generations) including sub-dermal and musculoskeletal treatment devices |
 Internal study of all cancer issues and health disorders of victims associated with 9/11 and other disaster-related environmental toxic exposures. Collaboration with geological labs & environmental statistics. (See First Responders Cancer Resource) Internal study of all cancer issues and health disorders of victims associated with 9/11 and other disaster-related environmental toxic exposures. Collaboration with geological labs & environmental statistics. (See First Responders Cancer Resource) |
 Function review / performance evaluation program of all laser-based medical equipment including devices specializing in sub-dermal musculatory treatment of chronic disorders. Function review / performance evaluation program of all laser-based medical equipment including devices specializing in sub-dermal musculatory treatment of chronic disorders. |





 QUANTIFIABLE DETECTION OF THERAPY RESPONSE
QUANTIFIABLE DETECTION OF THERAPY RESPONSE